Introduction:
The addition of rituximab to the treatment of CD20-positive (CD20+) Philadelphia chromosome (Ph)-negative acute lymphoblastic leukemia (ALL) patients has demonstrated considerable benefits in terms of event-free survival in a large randomized clinical trial, as well as overall survival (OS) in retrospective analyses. Consequently, it is now an integral part of the standard care chemotherapy regimen for these patients. Conversely, Ph-positive (Ph+) B-ALL patients have been excluded from these trials and are typically managed with tyrosine-kinase inhibitors (TKI) in combination with chemotherapy, steroids, or novel agents such as blinatumomab. However, the potential benefits and concerns of adding rituximab to CD20+ Ph+ B-ALL patients remain uncertain, as there are worries about prolonged immunosuppression and potential infectious complications.
Methods:
We conducted a multicenter retrospective study involving five tertiary care centers in Mexico. The study included patients older than 16 years with newly diagnosed Ph-positive B ALL between January 2009 and June 2022, according to the WHO criteria. CD20+ was defined as CD20 expression ≥20% by flow cytometry. The decision to administer rituximab was based on the physician's discretion. The primary objective was to compare the 3-year OS between CD20-negative patients and CD20-positive patients who received or did not receive rituximab, and as secondary objectives to evaluate infection rates and mortality during induction with the addition of rituximab.
Results:
The study comprised 119 patients who met the inclusion criteria. The median age at diagnosis was 38 years (16 - 73), with 52.1% being male. The median white blood cell count (WBC) count was 114 x 10 9/L (0.5 - 466). Of these, 45 patients (37.8%) had CD20+ B-ALL, and 24 of them (53.3%) received rituximab. A total of 111 patients (93.2%) received TKI treatment, with imatinib being the most common (79%), followed by dasatinib (13.4%) and ponatinib (0.8%). The majority of the patients received either Hyper-CVAD (41.2%) or a local adult regimen (31.9%). There were no significant differences in age, WBC count, or treatment/TKI between patients who received rituximab and those who did not. Table 1 presents the characteristics of the population based on CD20 expression and treatment. Early mortality was 5.8%, with no significant difference observed between patients who received rituximab and those who did not (0% vs. 7.4%; OR 0.78, 95% CI 0.71 - 0.86, p=0.342). The rate of neutropenic fever during induction was 72.3%, and it was lower in patients who received rituximab (50.0% vs. 77.9%; OR 0.28, 95% CI 0.11 - 0.72, p=0.010). The intensive care unit (ICU) admission rate during induction was 11.8%, with no significant difference observed between patients with or without rituximab (8.3% vs. 12.6%; OR 0.62, 95% CI 0.13 - 3.01, p=0.733).
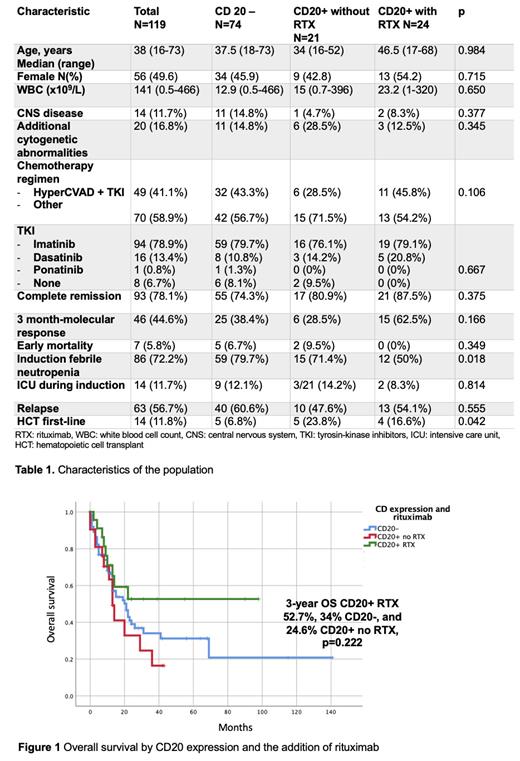
Molecular response at 3 months was measured in 72 patients and the use of rituximab was associated with a non-significant higher rate: 83.3% vs 57.4% with an OR of 3.71 (95% CI, 0.96-14.33, p=0.056).The median OS for the entire cohort was 20 months (95% CI, 13.34 - 26.65), with a 3-year OS of 35.7%. The leading cause of death was disease progression (77.3%), while 10.7% died in remission: 7.7% of CD20- patients, 21.4% of CD20+ patients without rituximab, and 11.1% of CD20+ patients receiving rituximab (p=0.436). There was a trend towards a better OS in CD20+ patients who received rituximab: median OS not reached vs. 21 months (95% CI 13.27 - 28.72) in CD20- patients, and 13 months (95% CI 9.7 - 16.29) in CD20+ patients without rituximab. The 3-year OS rates for these groups were 52.7%, 34%, and 24.6%, respectively (p=0.222; Figure 1).
Conclusions:
In real-world practice, rituximab is added to the chemotherapy regimen and TKI in 53.3% of CD20+ Ph+ B-ALL cases. Rituximab is not associated with a higher incidence of infections, ICU admission, early mortality, or deaths in remission. Although there is no significant benefit observed, there is a trend towards better OS with the addition of rituximab to the standard treatment of CD20+ Ph+ ALL. There is an unmet need to explore the concomitant use of rituximab and TKI in prospective studies.
Disclosures
Gomez-De Leon:Novartis: Honoraria; Abbvie: Honoraria; AMGEN: Honoraria; Astellas: Honoraria; Jnssen: Other: Advisory board; Sanofi: Honoraria. Delgado-López:Amgen: Speakers Bureau; Bristol: Speakers Bureau; Janssen: Speakers Bureau; Pfizer: Speakers Bureau. Demichelis:Pfizer: Consultancy, Honoraria; TEVA: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; AMGEN: Consultancy, Honoraria; Abbvie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal